Chromate Conversion Coating for Aluminum

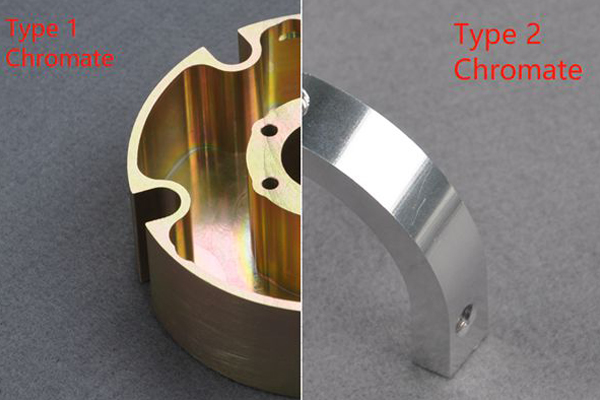

Coatings and Corrosion Protection
Chromate conversion coatings are used to passivate metals using an immersion bath process. The chromate conversion coating is a thin film that is applied by immersing the metal in a solution containing hexavalent chromium ions, which react with aluminum oxide on the surface of the metal to form a protective layer. This reaction forms a very stable complex with the chromium ion. The coating can be applied as either a single-stage or two-stage process. In the first stage, the metal is immersed in a dilute acid solution containing hexavalent chrome (Cr(VI)) ions, which reacts with the aluminum oxide to produce CrO3−2Al2O3.
During this step, some of the hexavalent chromium is reduced to trivalent chromium (Cr(III)). After rinsing, the coated metal is then dipped into a second solution containing hexavalent and/or trivalent chromium ions, usually at higher concentrations than those used in the first stage. This causes further reduction of any remaining Cr(VI) to Cr(III), forming additional CrO3−2 Al2O3. The resulting coating has a high concentration of chromium oxide, but it also contains residual amounts of hexavalent chromium.
Corrosion Control is a key benefit of Chromate Conversion Coating . Chromate conversion coating is one of the best methods available today for protecting aluminum from corrosion.
The following are the benefits of Chromate Conversion Coatings:
